WP1 Coordination and management
The aim of this deliverable is to describe the early project management activities which enabled successful project kickoff in May of 2020. Furthermore, it documents the initial project communication activities mostly related to informing the public about the project starting and roles different organisations will have in it.
Due to the travel limitations induced by COVID-19 pandemic the kick-off meeting was held in a virtual environment through a teleconference. Its organization and approval by the EC were predated by extensive work on risk assessment by each consortium member and as a group, as well as creation of a robust risk mitigation plan for the initial stages of the project. Once the project started the news was spread on the consortium member’s websites, social networks and in the local media.
Despite the challenges imposed by the pandemic the project has successfully started in May 2020 as originally intended. The consortium overcame the initial obstacles through collaboration and dedication to the common vision. The kick-off meeting was held and communicated to the public.
On this link you can find all the press releases, articles and media content related to consortium members and the project up to now.
WP2 Co-development, safety, and experimental deployment
Due date: 31.07.2023.
Actual submission date: 28.07.2023.
Partner responsible: ECF
Reviewed by: ST
The purpose of this deliverable is to present the list of co-development guidelines that have been drafted, drawing upon the valuable experience gained throughout the project. These guidelines serve as a comprehensive framework for fostering collaboration and interdisciplinary cooperation between technical partners and practitioners working in hazardous conditions.
The co-development process started with the questionnaires almost immediately upon the project start, where we identified the most important physiological variables, as judged by the first responders, and we checked sensors already available from the technological partners, for the Alpha demonstrator setup. The process has been continued during the whole development of the SIXTHSENSE project by means of meetings before and after the field tests and questionnaires submitted to the practitioners. After the tests of the Beta demonstrator we focused our attention on the existing references on guidelines for the co-development process in the field of first responders.
A set of 12 guidelines for establishing a successful co-development in a multidisciplinary environment have been drafted:Defining roles and responsibilities
- Engaging stakeholders early
- Clear definition of goals
- Establishing a collaborative environment
- Adopt a user-centric design approach
- Consider Usability and Ergonomics
- Consider Safety and Mistakes impact
- Collect end-users’ feedbacks and integrate them with technical possibilities
- Iterating and Prototyping
- Documenting the co-development Process
- Ethical Considerations
- Sharing Knowledge and Results
By leveraging the knowledge and expertise accumulated during our project, we aim to facilitate the successful implementation of co-development processes, ensuring the development of innovative solutions that address the unique challenges faced by practitioners in hazardous environments.
WP3 Non-invasive biosensors
Due date: 31.10.2021.
Actual submission date: 31.01.2021.
Partner responsible: TECSR
Reviewed by: TEC
This deliverable aims to report the first prototypes of sensing electrodes with integrated Organic Electrochemical Transistors (OECTs) and Interdigitated Microelectrodes (IMEs). It describes the key aspects of OECTs development: materials, geometries and functionalization of the channel.
Scheme of typical structure of an OECT.
This document shows the information in ALPHA stage for the development of prototypes of sensing electrodes with integrated OECT amplifiers and IME sensors. It will provide the technological capacity for the initial requirements of biomarkers detection such as cortisol and Na+ cations.
Prototypes of sensing electrodes with integrated OECT amplifiers developed by TECSR.
The alpha prototype OECTs and IMEs has been produced and delivered to the partners responsible for functionalization.
Due date: 31.01.2023.
Actual submission date: 31.01.2023.
Partner responsible: TECSR
Reviewed by: TEC
The deliverable aims to report the second iteration of prototypes of sensing electrodes with integrated Organic Electrochemical Transistors (OECTs). It describes the key aspects of OECTs development: materials and geometries.
The document shows the information regarding the various designs and materials used for the ALPHA prototypes of sensing electrodes with integrated OECT amplifiers and explains the reasoning and differences introduced with the revised version of the BETA sensors that resulted in a set of 40 different versions that will be produced and tested in the continuation of the project. The new set of sensors will provide the basis for benchmarking the various configurations and selecting the most appropriate configuration for biomarkers detection such as cortisol and Na+ cations.
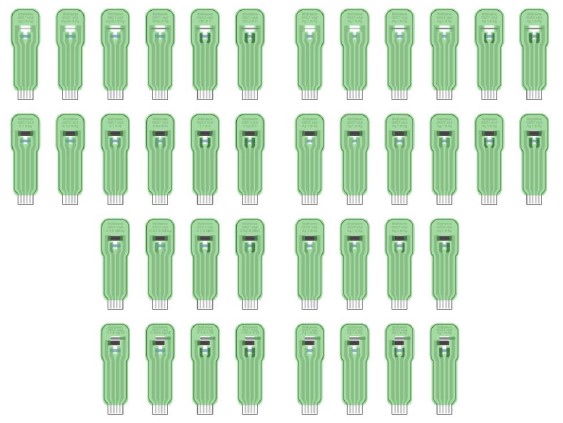
Technical drawing of 40 different BETA OECTs in which each layer/material is denoted with a corresponding colour: Ag – light grey, Ag/AgCl – dark grey, C – black, PEDOT:PSS – blue, insulation – green
Due date: 30.04.2023.
Actual submission date: 02.05.2023.
Partner responsible: MDS
Reviewed by: TEC
The main aim of this deliverable is to report the protocol of upscaling fabrication of all sensors developed along the execution of the project, preferable all integrated in one unique device. The key aspects of fabrication protocol are described as well as materials used for the fabrication of different devices. In addition, the costs of production are evaluated.
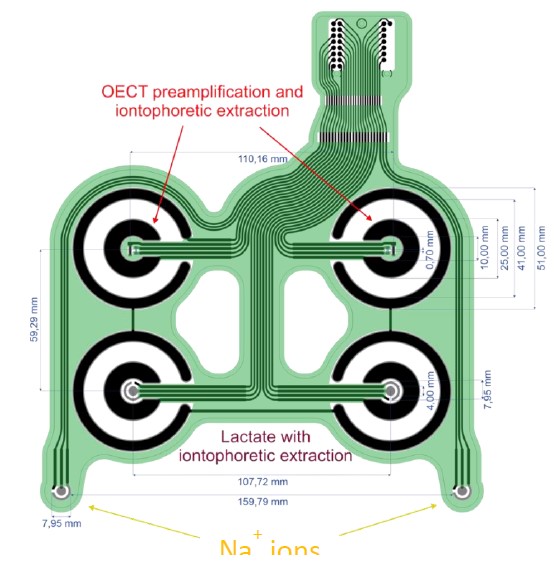
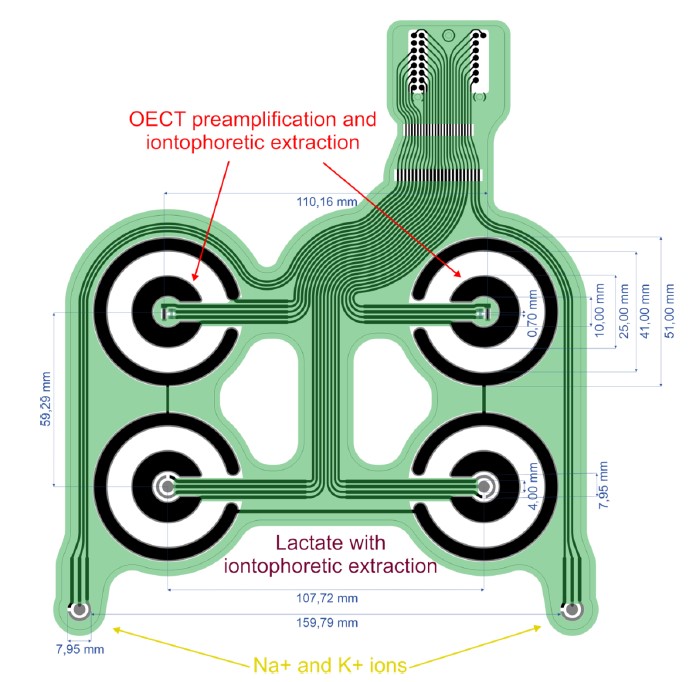
Final sensor platform integrating 6 sensors and 4 iontophoretic extraction systems
The final device integrates three different chemical sensors (cortisol, lactate and Na+), two of them with sensing phases based on biological recognition elements. The fabrication of the final device consists of two different manufacturing procedures. The first one is carried out using thick film technology. In this case the electrodes with different designs, that have been optimized during the project, are screen-printed on PET substrates using silver, carbon and PEDOT:PSS inks. After that, the second procedure is aimed to fabricate the different sensing phases. In this case the fabrication is carried out using inkjet printing technology. Finally, the cost of production is about 25 euros/device for high scale production, being this cost the minimum value that currently we can achieve due to the high cost of anti-cortisol antibodies.
WP4 Minimally invasive biosensors
Due date of deliverable: 31/10/2022
Actual submission date: 31/10/2022
Partner responsible for
this deliverable: 14 – BFS
Reviewed by: 3 – TUC
Aim
This deliverable summarizes the design, working principle, preliminary investigations, fabrication and characterization of a pumping mechanism which allows for sucking in a liquid into the sensor channel.
Approach
In contrast to the technology envisioned in the proposal phase, a novel concept for a suction pump was developed based on an ultrathin electroosmotic pump. This allows for a very flat and tiny device. The electroosmotic pump is based on a gold-coated track-etched membrane. For integration into the sensor device, the membrane is contacted by a ring electrode on the sensor substrate and an electrode-inlay on the opposite side, which is contacted back to the sensor substrate, so that only one connector is required.
Results
Final setup. a) Front and back view. b) Size comparison. c) Channel filled with blue water
The assembled sensor-pump setups were finally pre-filled with water and then pumped based on the results of the preliminary investigations.
Pumping of the full setup. The movement of the liquid front can clearly be seen and is
indicated by a red arrow. Almost no bubbles were generated on the gold-coated membrane.
Due date of deliverable: 31/10/2022
Actual submission date: 02/11/2022
Partner responsible for
this deliverable: 8 – JT
Reviewed by: 3 – TUC
Aim
The aim of deliverable 4.5 is to develop a way to sense analytes at very small flow rates. In the final application the interstitial fluid (ISF) to be measured is only available in small volumes and it can only be taken out of the skin with low speed (few µl/h). Since the enzymes in the sensor consumes lactate molecules, the actual concentration at the sensor becomes lowered for low flow speeds. Therefore a sensor setup is necessary that reduces the lactate consumption while keeping the sensitivity for the lactate in the ISF at a level useful for the transmitter electronics used. Reducing the sensitivity means reducing the lactate consumption and therefore decreases the minimum useful flow rate.
At low speeds also the effect of a chemical cross-talk within the sensor flow cell is greater and the H2O2 produced by the enzymes accumulates to higher levels and creates higher readings at the blank electrode of the sensor – in case the release of H2O2 into the ISF stream is not prevented by a catalase membrane topcoat. While without such catalase membrane the reading of the chemical cross talk at the blank electrodes can be used as an indicator of such ultra-low flow rates. An indication that is not possible by other means of lflow rate measurement.
Deliverable 4.5 is therefore an important step towards the final application.
Approach
The approach consists of these parts:
- Depositing functional membranes to the sensor electrodes by first adding molecular sieves by electropolymerization to the electrodes.
- Subsequently the enzymatic and diffusion control membranes will be dispensed and afterwards UV-cured.
- To minimize the lactate consumption and cross-talk the active area of the membranes should be as small as possible. The base idea is therefore to have small slits under the flow channel of the sensor. The slits form the cavity to hold the membranes as depicted in figure 5 creating band like narrow electrodes.
The challenge is hereby to optimize the membranes to be applicable by needle transfer dispensing to the slits and to flow into the slits forming the same proper setup as we have proven for larger cavities in deliverable 4.4. After the dispensing the subsequent solvent evaporation is challenging too since the greater ratio of the surface area of the slit side falls to electrode surface imposes special requirements to the membrane adhesion.
The sensor validation will be done in artificial ISF covering the full range of expected flow rates and range of concentrations of the ISF in the final application. Also testing of the most common interfering substances, such as acetaminophene and ascorbic acid, will be done.
The final sensor with the connector cannulas is shown below.
The sensor demonstrated in deliverable 4.4 could sense flows of down to 1 µl/hour, meaning in deliverable 4.5 we were able to reduce the measurable minimal flow by a factor of 20. It therefore can be stated that the goal of the deliverable was achieved.
WP5 Sensor data fusion
Due date: 31.01.2021.
Actual submission date: 28.01.2021.
Partner responsible: TECSR
Reviewed by: TEC
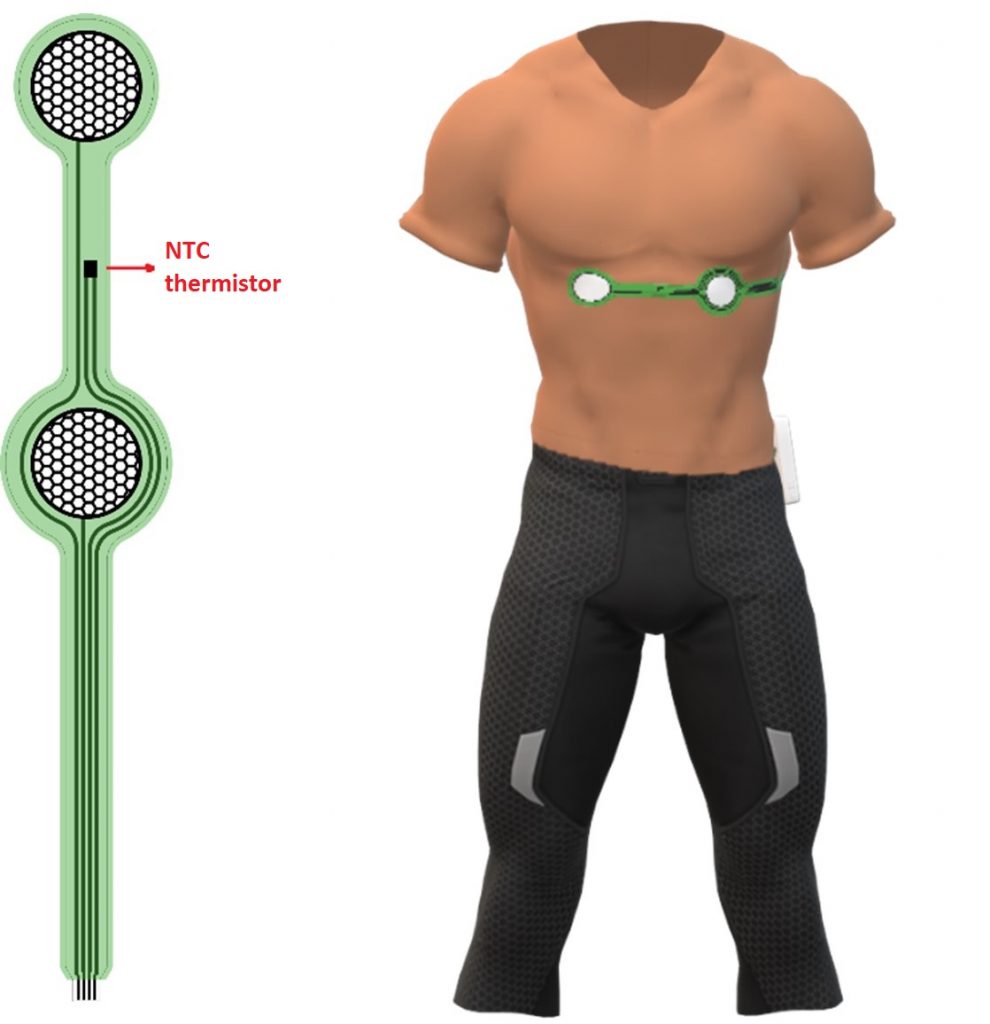
To the initial version of MEA basis for sensing based on the state-of-the-art sensors and physiological strain models. It contains information about the alpha prototype design, dimensions, manufacturing process and proposed positioning.
The alpha prototype of the MEA basis for sensing is based on the requirements defined in D2.1, focusing on the available technology and existing models for physiological strain.
The alpha version comprises a two lead ECG sensor and an NTC thermistor for measurement of the skin temperature. From the proposed design, advanced functional prototypes of MEAs will be iteratively developed to allow testing and validation in both laboratory and field conditions. Current design will serve as a test bed for further research and development, that will lead to a high level of integration of novel sensors to the existing architecture later in the project.
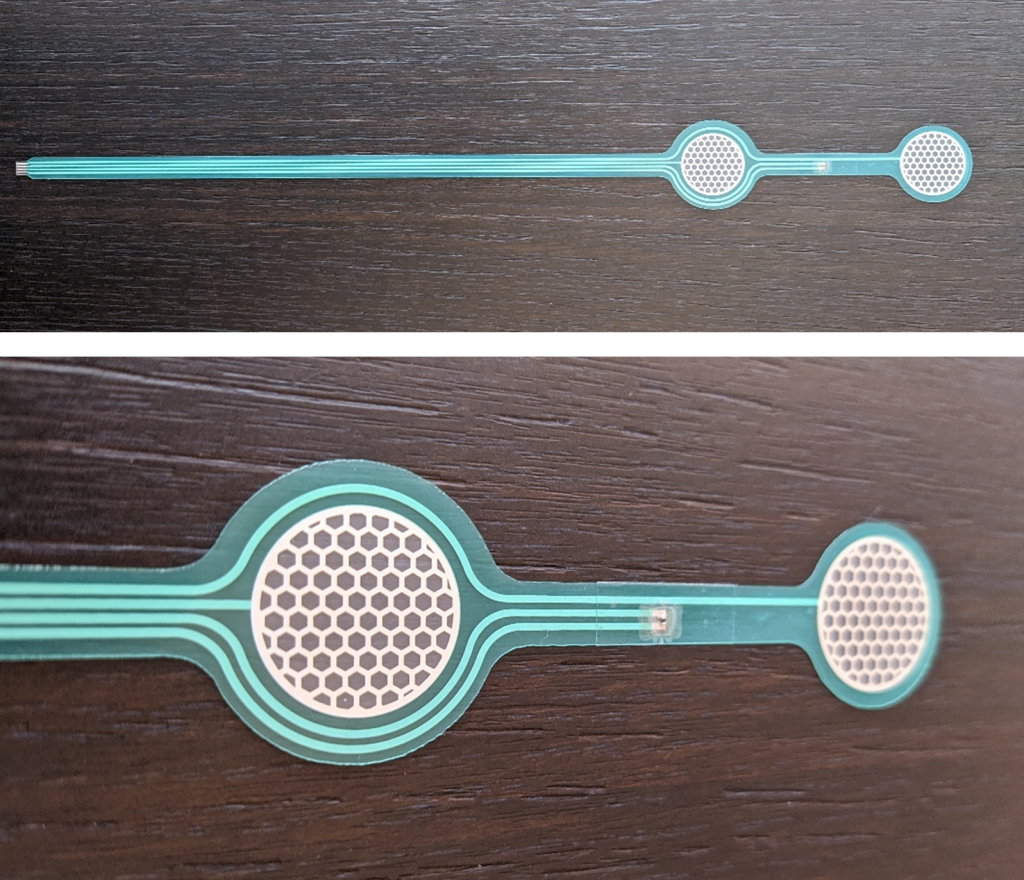
MEA basis prototype with NTC thermistor subsequently added (down).
First step towards fully integrated, single-substrate biosensing system was made by designing and manufacturing MEA basis with two ECG sensors and one NTC thermistor. The presented MEA basis is designed and developed using state of the art technologies and knowledge to be used for Alpha demonstration purposes.
Due date: 30.04.2021.
Actual submission date: 30.04.2021.
Partner responsible: GES
Reviewed by: SHG
To design and manufacture a prototype of integrated SIXTHSENSE embedded system which integrates data acquisition and electrical stimulation units. The design must comply with the requirements defined in D2.1, D7.1 and D9.1.
Based on the existing technology and GES knowhow a wearable health monitoring system with closed loop electrotactile biofeedback that that allows first responders in hazardous situations to sense their current health status was developed. The design was made in compliance with the modular architecture proposed in D9.1 that allows for it to be used in a standalone device, or as an integral module of the SIXTHSENSE wearable hub alpha prototype.
The developed Alpha mobile device is equipped with an ARM Microcontroller, acquisition system, electrotactile stimulation system, communication with command center, battery and interfaces. Set up of the equipment that was used for requirement verification is displayed in the image below.
The manufactured prototype fulfils all required characteristics, based on results of functional tests, as well as hardware photographs presented in this deliverable.
Due date: 31.10.2021.
Actual submission date: 31.10.2021.
Partner responsible: TECSR
Reviewed by: TEC
The aim of this deliverable is to serve as a supplementary document to the second version of SIXTHSENSE MEA basis for sensing prototypes, providing technical data on: configuration, shape and size of different sensors included, technical drawings, manufacturing process, proposed positioning.
The design of second version of SIXTHSENSE MEA can be seen in the image below. Beta prototype includes the following recording sites: 3 circular ECG electrodes (2 active and 1 reference pad), 4 standard 3-electrode cell configuration for chronoamperometric measurements of lactate, surrounded by two concentric electrodes for iontophoretic extraction, 2 standard 3-electrode cell configuration for open circuit potential (OCP) measurements of Na+ and K+ ions, NTC (Negative Temperature Coefficient) thermistor for temperature measurement.
The beta prototype of the Multi-Electrode Array (MEA) basis for sensing is optimized in terms of design and body site, based on the results of experiments for physiological strain assessment, as well as the technical validation of the alpha prototype. In this version, MEA basis includes sensors for measuring ECG, temperature, and concentration of three biomarkers: lactate, sodium cations (Na+) and potassium cations (K+), and it is envisioned to be placed on the back (as shown in the image below).
The presented MEA basis for sensing is designed and developed to be included in Beta demonstrator. Current design will serve as a test bed for further research and development, that will lead to a high level of integration of novel sensors to the existing architecture in the Gamma stage of the project.
Due date: 31.07.2022.
Actual submission date: 02.08.2022.
Partner responsible: GES
Reviewed by: SHG
The aim was to design and manufacture a prototype batch of Sixthsense embedded systems, which integrate data acquisition and communication means. The design must comply with the requirements defined in D2.4, D5.4, D8.2 and D9.3.
Based on the existing technology and GES knowhow a wearable health monitoring system with closed loop electrotactile biofeedback that allows first responders in hazardous situations to sense their current health status was developed. The design was made in compliance with the modular architecture proposed in D9.3 that allows for it to be used in a standalone device, or as an integral module of the SIXTHSENSE wearable hub beta prototype.
The Beta system integrates two independent units: the Beta Acquisition Device (BACQ) and the Beta stimulator (BetaStim). This architecture provides galvanic isolation which is very important for proper data acquisition and system operation. BACQ is used for multimodal data acquisition, iontophoresis, control of the BetaStim over the Bluetooth communication link and for RF communication with command centre.
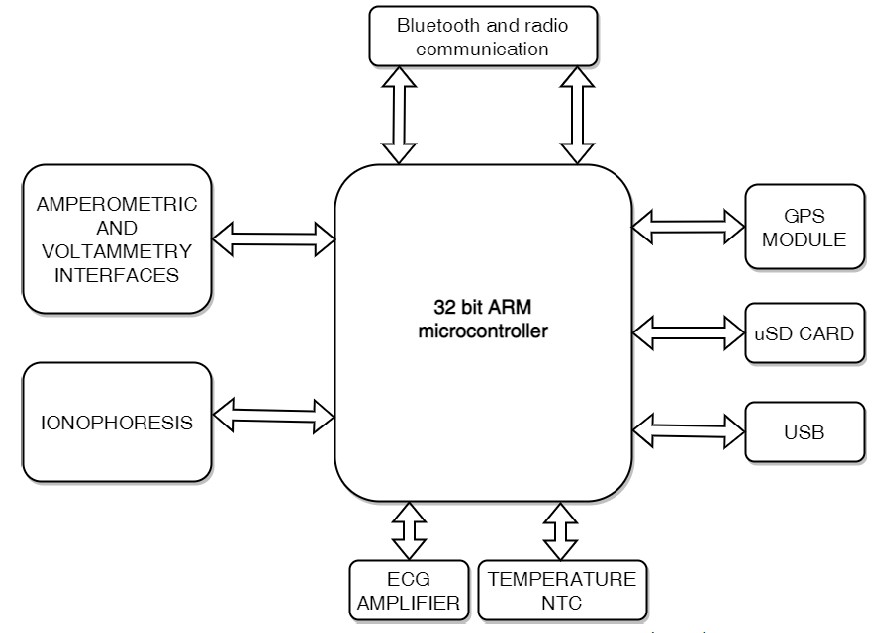
Block diagram of the Beta Acquisition Device (BACQ)
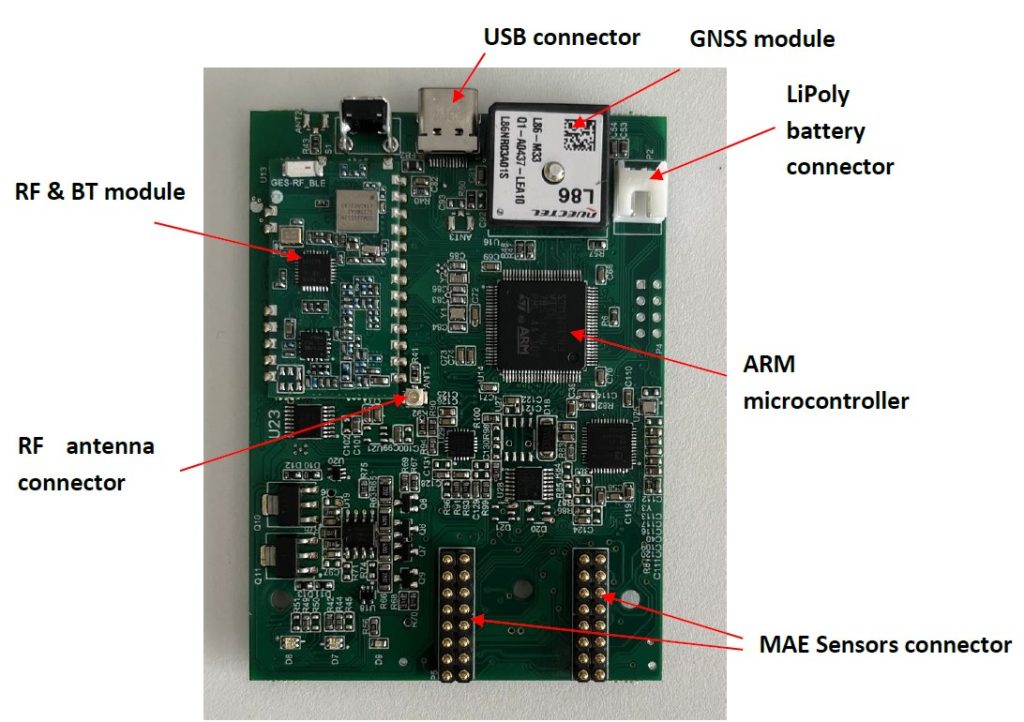
Manufactured BACQ PBC
Due date: 31.01.2023.
Actual submission date: 30.03.2023.
Partner responsible: TECSR
Reviewed by: TEC
The aim of this deliverable is to serve as a supplementary document to the third version of SIXTHSENSE MEA basis for sensing prototypes, providing technical data on:
- Configuration, shape and size of different included sensors
- Technical drawings
- Manufacturing process
- Proposed positioning.
The gamma prototype of the Multi-Electrode Array (MEA) basis for sensing is optimized in terms of design, based on the results of experiments for physiological strain assessment, as well as the technical validation in laboratory of the beta prototype. In this version, MEA basis includes sensors for electrochemical detection with and without iontophoretic capabilities, following the same concept used in Beta prototype. The novelty in this stage is the embedding of organic electrochemical transistors (OECTs) for achieving a superior transducing ability based on the coupled nature of ionic and electronic fluxes. In this stage the ECG and temperature sensors are omitted from the design of non-invasive electrochemical MEA gamma prototype.
Technical drawing of the Beta prototype MEA for sensing with labelled dimensions.
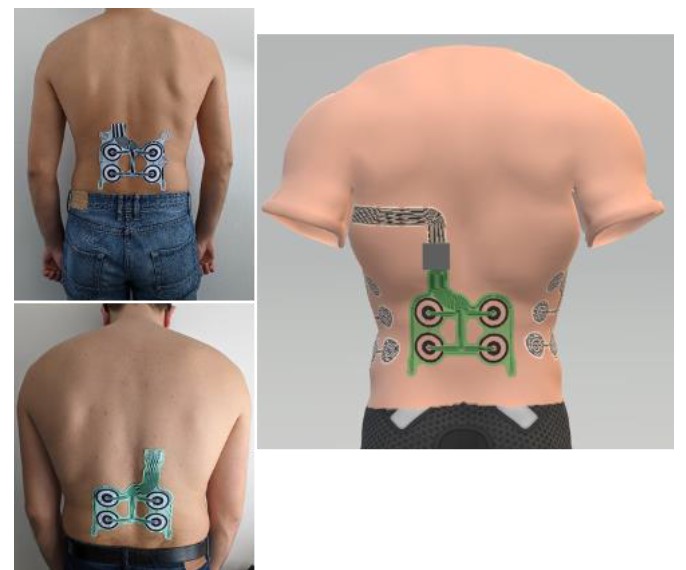
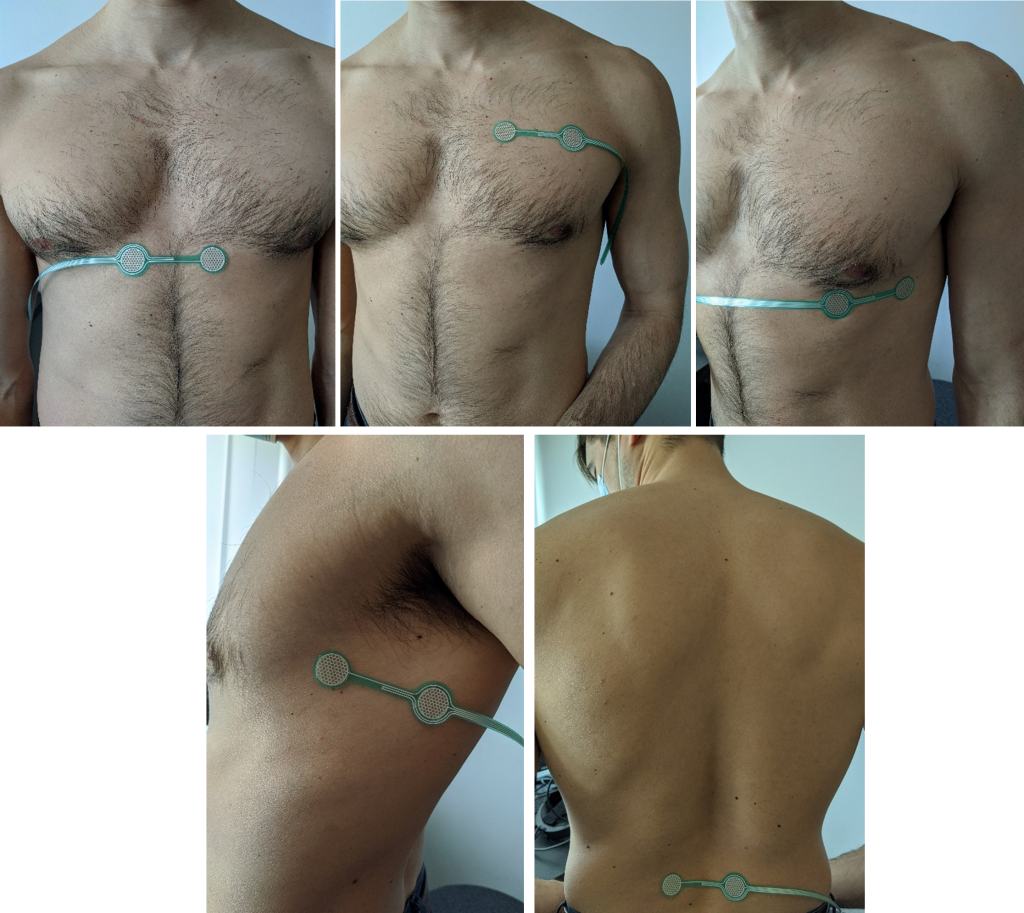
MEA basis positioning: Beta (top left image), Gamma (bottom left image), Gamma system vision including sensing and stimulation electrode locations on a 3D torso model (right image)
Gamma prototype of the MEA basis will be embedded in a garment that ensures intimate and secure contact with the skin in order to accurately detect all electrochemical parameters related to physiological strain, as it was proposed in beta stage. It is designed and developed to be included in the gamma demonstrator but is also backward compatible with the beta devices which is used as test bed in this stage.
Due date: 31.07.2023.
Actual submission date: 31.07.2023.
Partner responsible: SHG
Reviewed by: TECSR
To design and manufacture a prototype of integrated Sixthsense embedded system which integrates data acquisition and electrical stimulation units. The design must complies with the requirements defined in D2.1, D7.1 and D9.1 and is based on the alpha and beta prototypes as described in D5.3/D5.6 and experiences of corresponding lab and field trials.
Based on the existing ALPHA and BETA prototype, it was necessary to develop it further in the next iteration, in particular to enable the integration of new or additional components and to improve signal quality, both for the acquisition and RF data transfer. The design was made again in compliance with the modular architecture of proposed in D9.1 that allows for it to be used in a standalone device, or as an integral module of the SIXTHSENSE wearable hub alpha prototype. Based on the existing alpha and beta prototype technology and GES knowhow a wearable health monitoring system with closed loop electrotactile biofeedback that allows first responders in hazardous situations to sense their current health status was developed. The design was made in compliance with the modular architecture of proposed in D9.1 that allows for it to be used in a standalone device, or as an integral module of the SIXTHSENSE wearable hub alpha prototype. The further development was carried out by SHG and was primarily aimed at the integration of essential features, which were increasingly aimed at confomity with regulatory requirements. Furthermore, minor changes were made to correct identified errors. The overriding goal, however, was to develop the GACQ (Gamma Acquisition Device) efficiently within the constraints of the possibilities and difficulties of the supply chain, without taking too many risks.
Exploded-view Drawing of GACQ (left). GACQ with enclosure (right). The lid for the Pogo-Pins can be closed when with the connector present.
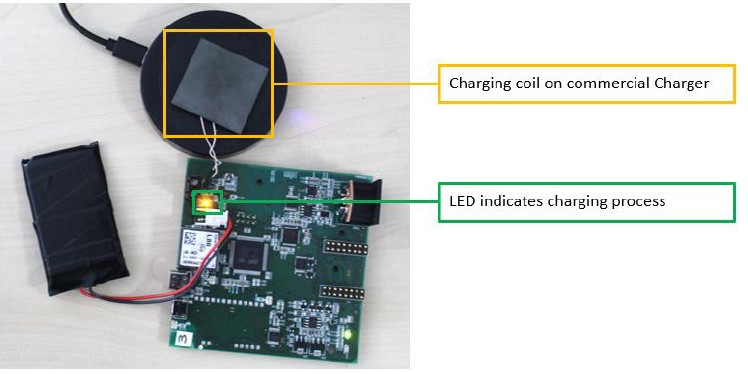
Testing wireless charging capabilities
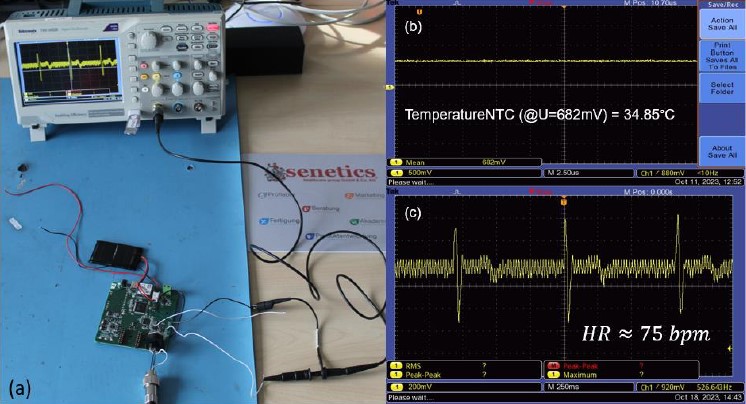
Test setup (a) of the NTC (b) and ECG (c) signal readings from the sensory vest (vest worn by voluntary)
Despite all the adversities and partly unfavorable circumstances (e.g. COVID, supply chain), the technology transfer from GES to SHG was successfully implemented and a functionally operational GACQ was developed. The manufactured prototype fulfils all new requirements and was tested in lab conditions primarily and technical features were verified during the field trials in Rijeka, Sept. 2023.
WP6 Data analytics and DSS
Due date: 29.01.2021.
Actual submission date:
Partner responsible: JR
Reviewed by: TEC
The aim of this deliverable is to provide a basic data management infrastructure useable by all project partners with low integration effort suited for the alpha demonstrator.
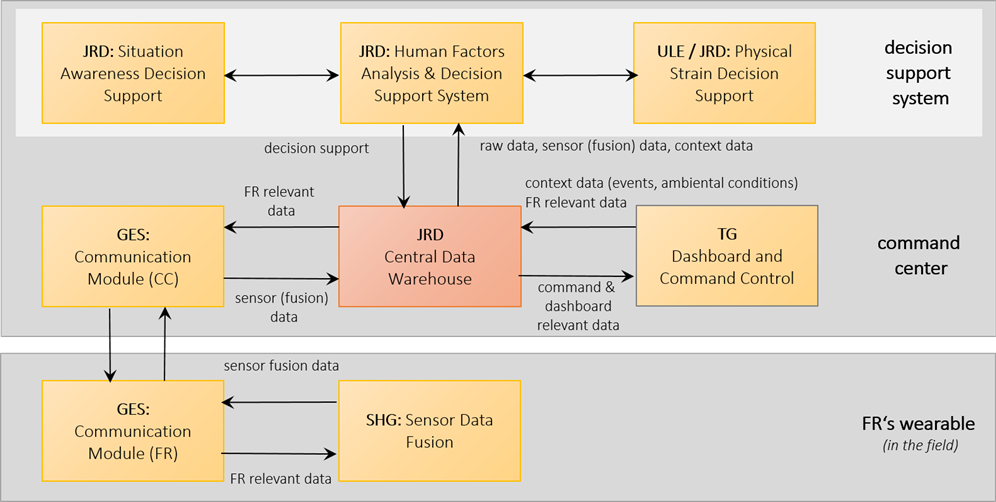 Overview of the basic data communication flow.
Overview of the basic data communication flow.
A Central Data Warehouse (CDW) has been implemented which is accessible for all project partners with well-defined and simple to integrate access clients as well as interfaces. Those client software components, as well as the interfaces have been thoroughly unit tested before internal distribution. The basic data management supports all needed functionalities as required by the alpha demonstrator. Lessons learnt in the first test stage will be integrated into the data management as further optimizations.
The multidisciplinary nature of the project partners within SIXTHSENSE, their differences in experiences in data handling and processing showed the need for a common basic data infrastructure. In the course of the specification of the Central Data Warehouse (CDW) it became apparent that by simplifying access to data and by providing sample sets, development on tasks depending on this data can be parallelized. Also, in order to not only rely exclusively on field trials for data generation, a simulator for bio–signals has proven to be beneficial. Further improvements of the data management infrastructure will extend the back channels from the decision support system and the command centre via the CDW to the first responders in the field.
WP7 Human machine interfaces
Due date: 31.01.2021.
Actual submission date: 28.01.2021.
Partner responsible: GES
Reviewed by: TECSR
The aim of this deliverable is to design and prototype an electrical stimulation unit that complies with the requirements defined in previous deliverables (D2.1, D7.1 and D9.1).
Based on the existing technology and GES knowhow a multichannel electrical stimulation prototype was developed. The design was made in compliance with the modular architecture proposed in D9.1 that allows for it to be used in a standalone device, or as an integral module of the SIXTHSENSE wearable hub alpha prototype.
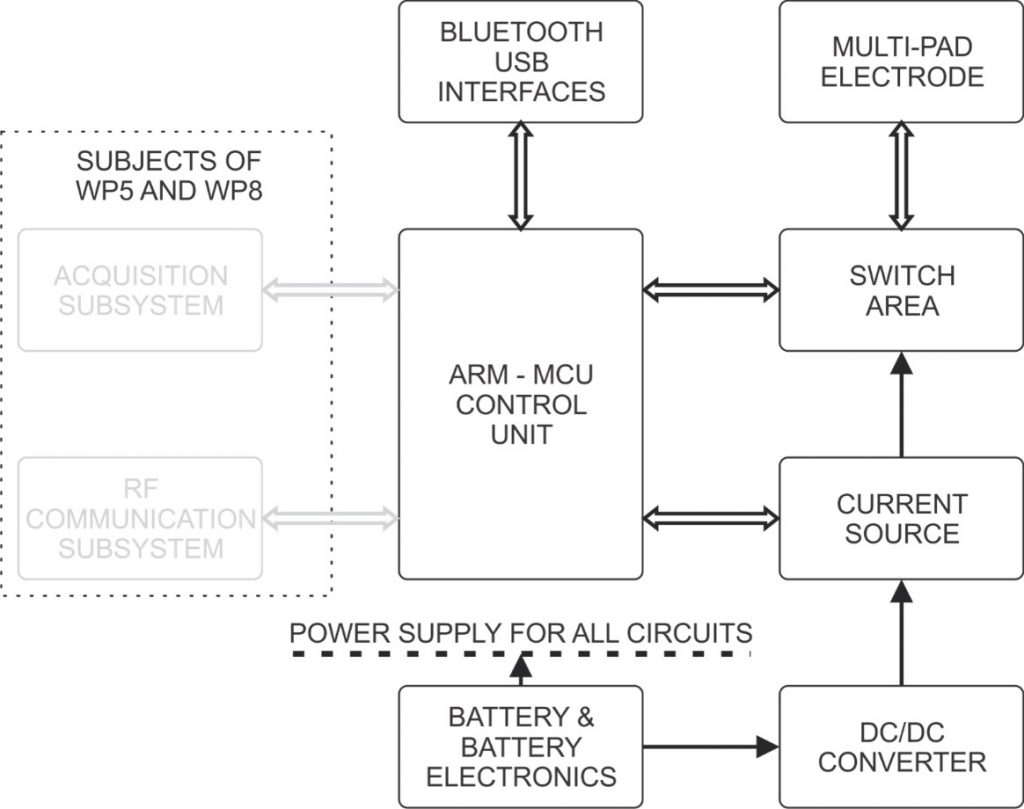
Block diagram of the Alpha electrotactile stimulator.
The manufactured prototype fulfils all required characteristics, as can be seen from results of the functional test, as well as hardware photographs presented below.
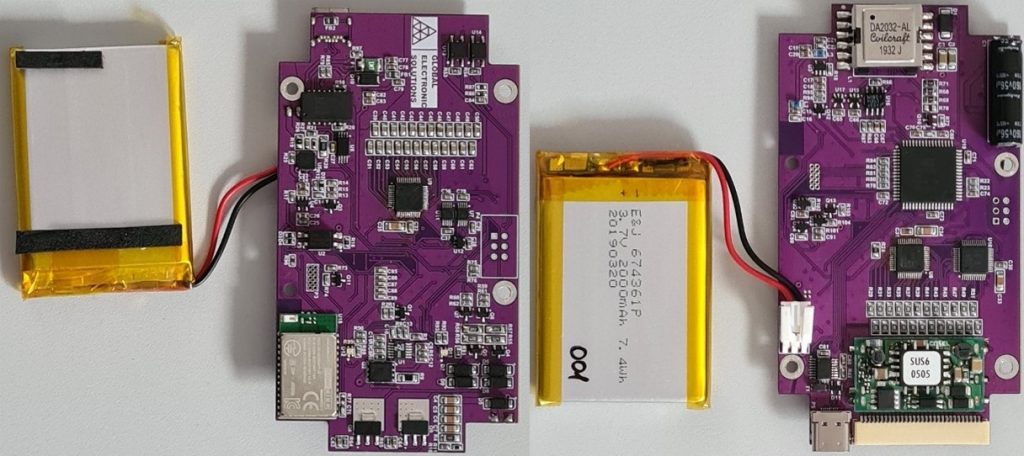
Realized stimulator prototype: top side (left), bottom side (right).
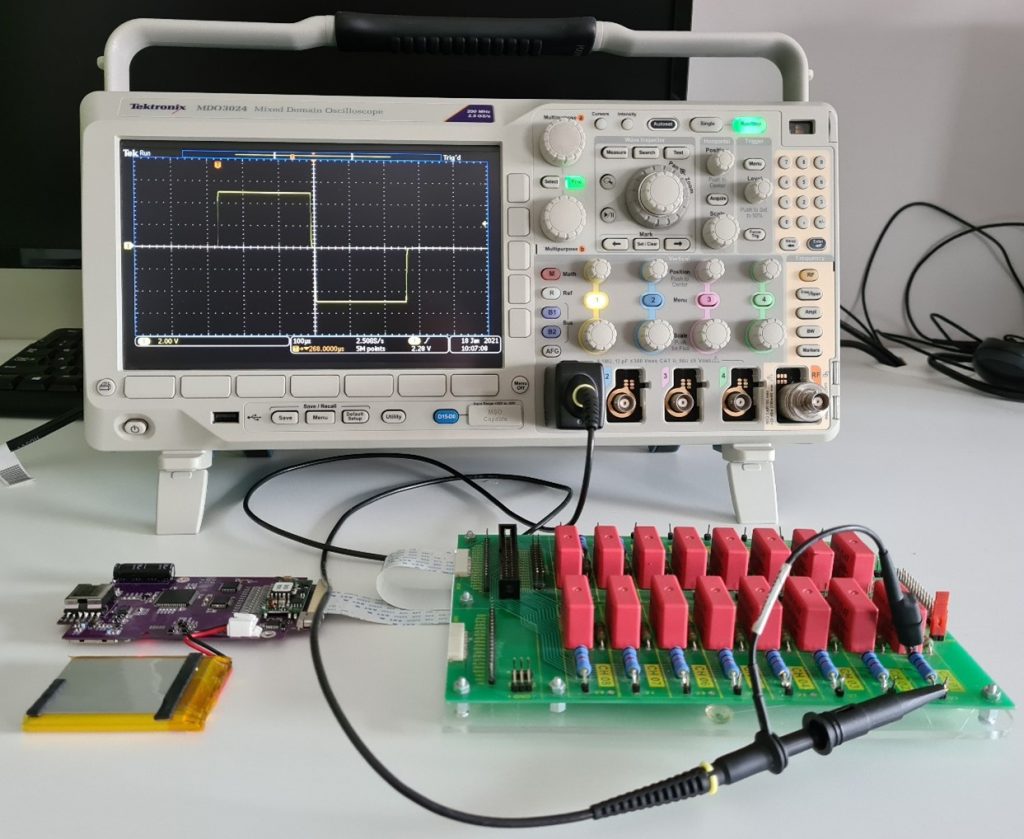
Main set-up for measurement tests. Stimulator output is connected to a Human Model Equivalent Circuit where current controlled pulses are converted into the signals that are easily measured with the oscilloscope.
An example of the test results for the Alpha stimulation system can be seen in the picture below. For this test, the oscilloscope was connected to HME circuit as shown in picture above, and its input was set to DC. The stimulator prototype was set to generate biphasic charge compensated pulses with pulse width set to 250µs, pulse rate to 50 pps, and amplitude to 1 mA.
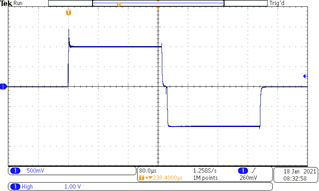
Resulting voltage obtained during 1 mA pulse current test. Time base is set to 80 µs/div and vertical sensitivity to 500 mV/div (0.5 mA/div).
Due date: 31.01.2021.
Actual submission date: 28.01.2021.
Partner responsible: TECSR
Reviewed by: AAU
The aim of this deliverable is to serve as a supplementary document to the first version of SIXTHSENSE electrode prototype, providing technical data on:
- Shape, size, configuration, number of pads and pad distribution;
- Technical drawings;
- Manufacturing process;
- Proposed positioning.
In order to provide reliable electrotactile feedback through discrete and continuous messages, a design containing two larger (top and bottom) and six smaller pads in 3×2 matrix configuration is proposed.
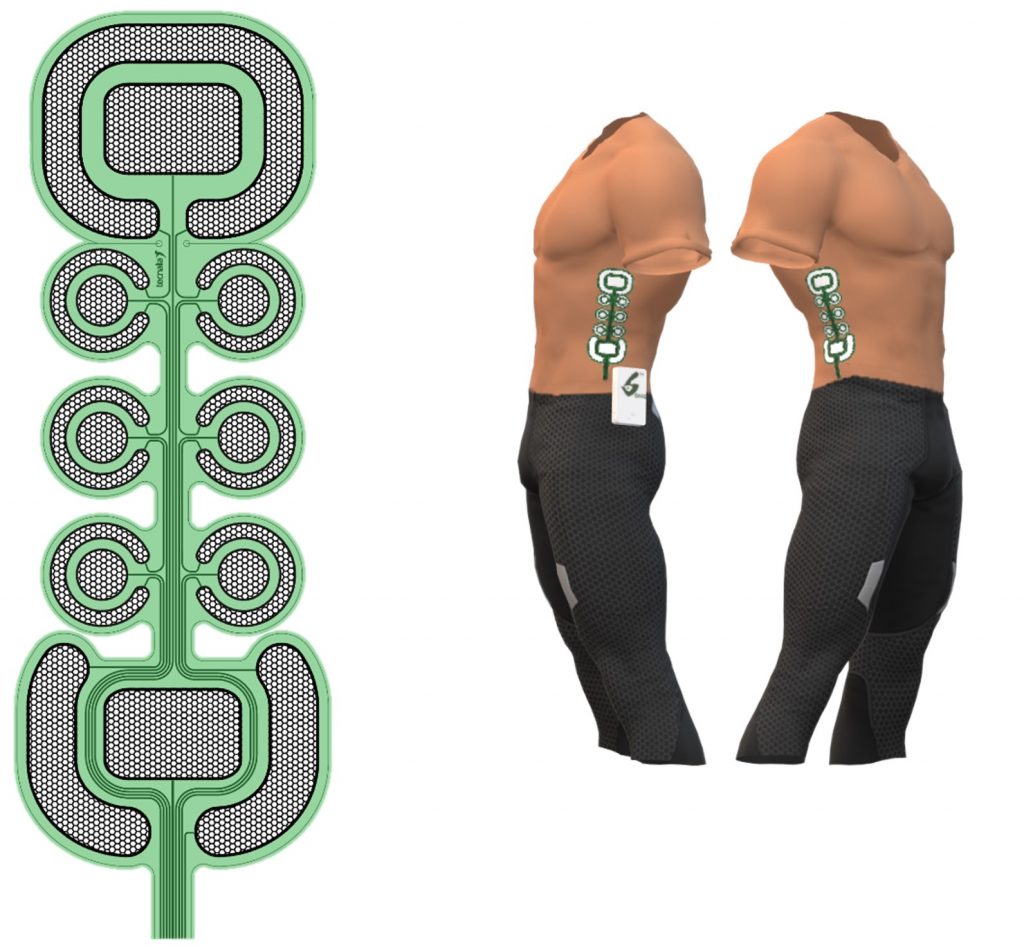
Technical drawing of the electrode design (left) and positioning of the electrode and the stimulator unit illustrated from two different viewing angles of 3D body model (right). The stimulator (white box) can be worn around the waist.
The electrode prototype is manufactured using well-known, state–of–the–art materials and technologies, while being conscious of the fact that it needs to be incorporated in a wearable garment such as a vest. Silver/silver-oxide conductive inks are screen printed on PET based substrates, and hydrogel for electrical stimulation is used as skin/electrode interface.
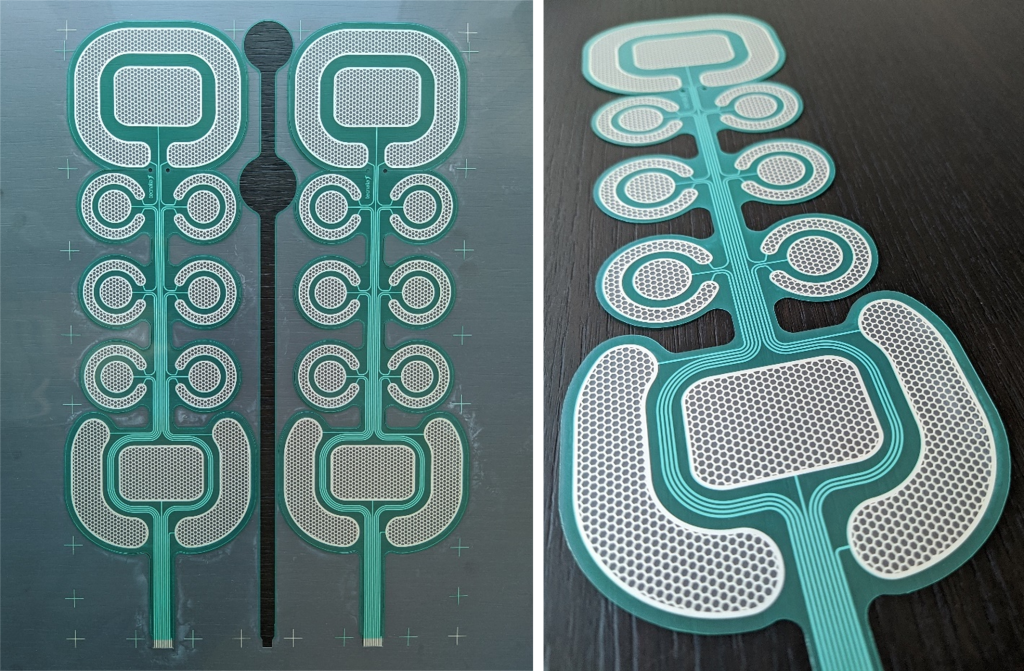
Screen-printed electrode design.
Subsequent electrode prototype designs will be optimized based on the further results of psychometric experiments and technical validation.
The presented electrode prototype is designed and developed using state–of–the–art technologies and knowledge and will be used for Alpha demonstration purposes. Based on pilot trials the preferred option for the electrode position is the lateral side of the torso on both right and left body side.

Electrode positioning on the lateral torso.
Due date: 31.10.2021.
Actual submission date: 31.10.2021.
Partner responsible: TECSR
Reviewed by: AAU
The aim of this document is to serve as a supplementary document to the second version of SIXTHSENSE stimulation electrode prototypes, providing technical data on: shape, size, spatial configuration, number of pads and pad distribution, technical drawings, manufacturing process and proposed positioning.
The initial version of the SIXTHSENSE stimulation electrodes (presented in D7.3) contained two larger (top and bottom) and six smaller cathode pads in 3×2 matrix configuration, each surrounded by a concentric anode. In this version (Beta prototype), two different electrode designs are presented. Both designs include 8 equal cathode pads distributed in a squared shape, i.e., in a 3×3 matrix without a central pad. The designs differ in terms of anode configuration (concentric vs common, displayed as left and right in the image below). The electrode prototypes are screen-printed using stretchable inks, which allows simple integration in a wearable garment such as vest.
Technical drawing of two proposed electrode designs.
The envisioned positioning of the SIXTHSENSE electrotactile stimulation electrode on the lateral torso was tested during Alpha experimental and field trials and it was decided to let it remain the same for the beta stage, and Beta electrode prototypes were therefore designed to be positioned along both the left and right sides of the lateral torso.
Two designs of the Beta electrode prototype were presented, which will be used to study the differences in anode configuration and size. Based on the subsequent results of psychometric evaluation, the preferred design (or both of them, if no preference is identified) will be used for Beta demonstration purposes. Additionally, the electrode prototype design will be further optimized based on the results of psychometric experiments and technical validation.
Due date: 31.01.2022.
Actual submission date: 01.08.2022.
Partner responsible: GES
Reviewed by: TECSR
The aim of this deliverable is to design and manufacture a prototype batch of Sixthsense electrotactile stimulation units that can meet the needs of further research within the WP7 and WP2 activities. The design must comply with the requirements defined in D2.4, D8.2 and D9.3.
The Beta stimulation system was designed following the good practices already established with the Alpha version. There are two major improvements in the architecture. First, it is designed as a galvanically separate module from the recording device, to allow simultaneous recording and stimulation. The implementation in this stage goes a step further, and physically separates the two devices, to allow greater flexibility in experimental design and execution of laboratory and field tests. Second, the physical interfaces to the stimulation electrodes is changed from ZIF to custom made pressure connectors, which allows stable connection with the textile embedded electrodes developed within the WP7 and WP5 efforts.
The Beta stimulation unit consists of the following blocks:
- DC/DC step-up voltage converter
- Biphasic current source
- Switch area (output de-multiplexer)
- Control unit
- Battery and interfaces
The interconnections between blocks are shown in Figure 1. In Figure 2 the photos of the realized stimulator prototype are presented.
Block diagram of the Alpha electrotactile stimulator.
The manufactured prototype fulfils all required characteristics, as can be seen from results of the functional test, as well as hardware photographs presented below.
Realized stimulator prototype: top side (left), bottom side (right).
Main set-up for measurement tests. Stimulator output is connected to a Human Model Equivalent Circuit where current controlled pulses are converted into the signals that are easily measured with the oscilloscope.
An example of the test results for the Beta stimulation system can be seen in the picture below. For this test, the oscilloscope was connected to HME circuit as shown in picture above, and its input was set to DC. Time base is set to 80µs/div and vertical sensitivity to 2V/div (2mA/div). The stimulator prototype is set to generate biphasic charge compensated pulses, with the following parameters:
• Pulse width: 250µs
• Pulse amplitude: 5mA
• Pulse rate: 50pps
Resulting voltage obtained during 1 mA pulse current test. Time base is set to 80 µs/div and vertical sensitivity to 500 mV/div (0.5 mA/div).
Approach
Implementation of the SIXTHSENSE dashboard for operation monitoring presented in this deliverable was based on the dashboard specifications from D7.5 Specifications for feedback for first responders – Beta.
Findings and results
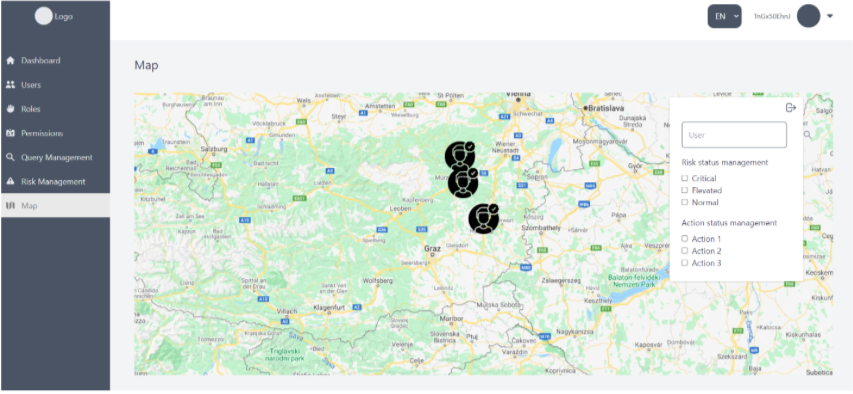
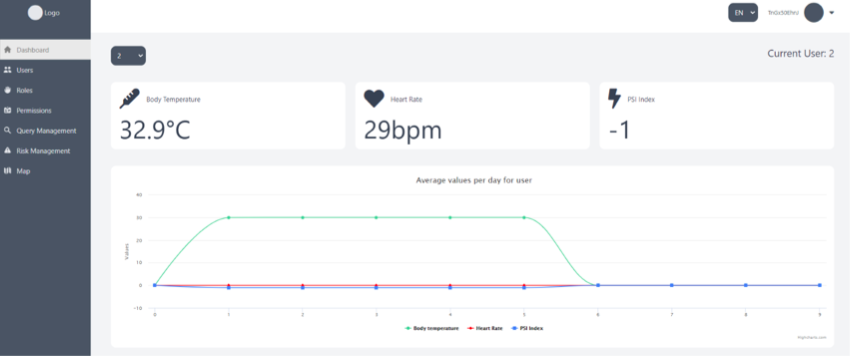
The presented SIXTHSENSE dashboard includes five modules:
1. Dashboard landing page,
2. Query management,
3. Risk management,
4. User management,
5. Map.
This document describes features of each module/page and provides instructions for end-users.
Following the technical validation, SIXTHSENSE dashboard will be tested in field-trials with first responders. Based on the first responders’ feedback and further developments of the SIXTHSENSE health monitoring device, the dashboard structure will be updated and presented in D7.14 Integrated command centre dashboard.
A public demonstrator of the SIXTHSENSE dashboard presented in this deliverable is available the link https://sixthsense.telegroup-ltd.software/.
To access please use the following credentials:
Username: admin@test.com
Password: Pass.123
It includes a mock dataset to allow visitors to test supported features.
Due date: 30.04.2023.
Actual submission date: 26.06.2023.
Partner responsible: TECSR
Reviewed by: AAU
This deliverable aims to serve as a supplementary document to the third version of SIXTHSENSE stimulation electrode prototypes, providing technical data on:
- Shape, size, spatial configuration and pad distribution;
- Technical drawings;
- Manufacturing process;
- Proposed positioning.
The Alpha version of the SIXTHSENSE stimulation electrode and one version of the Beta stimulation electrode had active pads surrounded by concentric referent pad (presented in D7.3 and D7.6). The second version of the Beta electrodes had the referent pad with the larger area relative to the active ones in the middle. Tests showed that this referent pad could be used as an information carrying pad when all other pads were activated synchronously.
Hence, the Gamma electrode consisted of 18 pads (16 active and 2 referent) in 3 x 6 configuration. The electrode prototypes are screen-printed using stretchable inks, which allows simple integration in the Gamma garment.
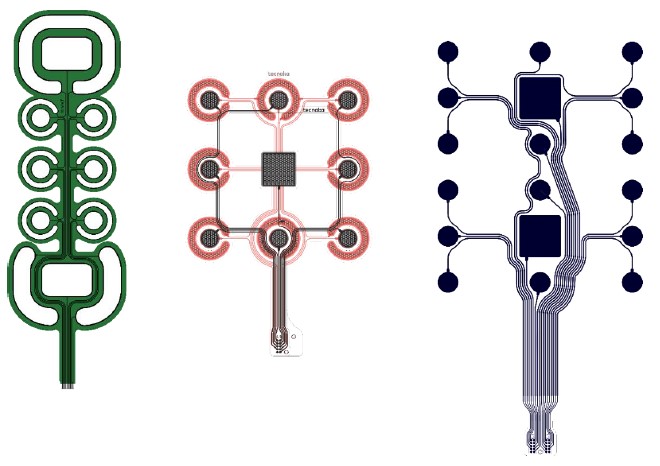
Comparison of the three iterations of the SIXTHSENSE stimulation electrode prototypes (green – Alpha, black/red – Beta, navy – Gamma). The electrodes are aligned at the middle of the active part of the electrode.
The novel design enables electrotactile sending of the messages through a higher number of electrode pads. The psychometric evaluation will show how and does the increase in electrode pad resolution contribute to smoother dynamic electrotactile messages transition and easier messages recognition. The increased number of the pads will increase the current calibration procedure, so only the relevant pads will be used for the Gamma demonstration purposes.
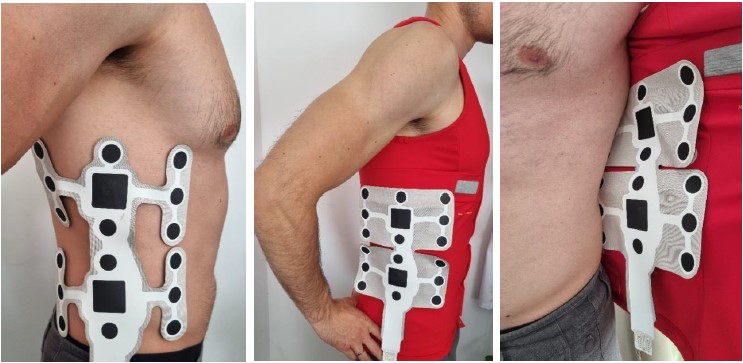
Gamma electrode (left) illustrated the position of the electrode on the skin, (middle) illustrated the position of the electrode on the upside-down Gamma garment and (right) position within the Gamma garment.
Due date: 30.04.2023.
Actual submission date: 02.05.2023.
Partner responsible: TG
Reviewed by: TECSR
Starting from the SIXTHSENSE dashboard for operation monitoring presented in D7.9 this deliverable presents the update that includes the feedback provided by the first responders during the co-development sessions and notes made in deployments throughout the Beta stage of the project.
A public demonstrator of the SIXTHSENSE dashboard presented in this deliverable is available the link https://sixthsense.telegroup-ltd.software/ . To access please use the following credentials:
Username: admin@test.com
Password: Pass.123
WP8 Mission critical telecommunications
WP9 System integration & validation
Due date: 30.04.2021.
Actual submission date: 30.04.2021.
Partner responsible: ST
Reviewed by: TECSR
The Alpha system combines different sensing modules in a redundant configuration on both physical and data level. This platform has been conceived to allow the sharing within the consortium of a common vision on the SIXTHSENSE potentiality, on the base of a practical experience. Parts of the developed Alpha system will be integrated in the demonstrator that will allow laboratory and field testing.
The starting point was the Alpha architecture described in deliverable 9.1 (and presented partly in the image above) where a detailed description of each sensing module combined with the technological asset have been reported by the different partners. From this list, modules that were ready for integration in the Alpha demonstrator were selected and integrated into the alpha prototype.
Modules that were ready for integration are the following: AMD (displayed in the image above), electrotactile electrodes and NTC sensors (positioning displayed in the image below).
The proposed system represents a solution in which the technological assets brought into the project by different partners has been integrated to evaluate in relevant conditions the soundness of preliminary approaches and the interplay of different modules, all in compliance with the common vision of SIXTHSENSE.
The platform described will allow the testing of each sensor or module. The Alpha prototype does not foresee the use of all sensors which are being developed in the project, but will focus on the set that is on a higher TRL and will allow field tests in the second year of the project. The sensors and modules on lower TRL will be tested in laboratory conditions, by using the same equipment (Alpha mobile device) but outside the presented functional prototype.
Partner responsible: ST
Reviewed by: TECSR
The BETA system combines different functional modules developed on the base of Alpha platform experience. The new platform has been conceived to provide a high-level architecture and identifies the existing interface standards that may be used to integrate a wide variety of technologies that were previously available and/or developed during the Alpha stage.
Starting from the BETA architecture described in deliverable 9.3 where a detailed description of each functional module combined with the technological asset have been reported, the BETA demonstrator has been implemented and tested.
Preliminary results on Alpha system and advancements on the development of the technological WPs have been considered to design the Beta garment version. The platform integrates new sensors that have to be in close contact with the skin. Rijeka tests shown that the Alpha T shirt was not enough to guarantee a good contact of the MEA during activity. This was observed despite the use of a shaping and compressive fabric. For this reason, a different solution was envisaged, the Beta version is based on an elastic stretchable garment, also if this solution can be perceived less performant in term of comfort and usability, as confirmed by the questionnaire results, collected during the Bormio trials, this functional version is considered as a mandatory step to validate the integration of the sensors and develop a comfortable vest for the Gamma platform. Design started with the materials selection, a very innovative elastic structure has been selected and different mock-ups have been realised to test functionality and material performances, as well as to assess wearing requirements during Bormio field trials. The first responders that participated in Bormio trials, have been considered as a focus group for the design process.
The garment is formed from 2 rectangular straps joined in the middle section to form a stretchable belt as shown in the figure below. The material used is stretchable in one direction, so that the garment can be stretched around the body, but the vertical dimension is stable. One side of the material is smooth, while the other is fuzzy, allowing it to work as the “loop” component of a hook-and-loop closing mechanism (velcro).
There is an orange line sawn down the middle of the garment, serving as a simple indicator for symmetrical positioning of the belt on the torso.
The following image shows the first BETA prototype with the indication of the position of the sensors and actuators.
Partner responsible: ST
Reviewed by: TECSR
The GAMMA version represents a streamlined version of the SIXTHENSE system focusing on usability and robustness, for effective use in relevant conditions of the environments where the first responders need to operate.
The sensors for ECG and temperature measurement are embedded in a fitted vest that adheres to the users body, while allowing full range of unhindered movements and being is simple to apply and remove. Segments that hold the electrotactile electrodes and multielectrode array of sensors for electrochemical sensing are especially designed to feature padding to ensure intimate connection with the user’s skin even in high intensity physical activity.
The first version of the gamma prototype has been piloted with mountain rescuers in the Kopaonik field trials in March 2023.
Check it out in this short video:
WP10 Dissemination, exploitation and communication
Due date: 31.07.2021.
Actual submission date: 30.07.2021.
Partner responsible: MDS
Reviewed by: TEC
The main aim of this deliverable is to establish an initial engagement with SIXTHSENSE project stakeholders. Alpha prototype is presented to main stakeholders identified by the project partners with the aim to receive feedback and influence on the achieved technology development. The early-stage involvement of external partners will enhance the number of sectors where this innovative technology could be applied.
In this document, the main information related to the preparation and execution of Alpha stakeholders’ workshop is reported. The initially planned face to face event in a parallel session of a Conference was changed to an online workshop, with several talks and demonstration videos. The selected group of stakeholders were invited to participate in the workshop and smooth and effective communication pathways were established for the future. Main outcomes and feedback provided by the stakeholders are collected and will be considered for a better implementation of the SIXHTSENSE project and future stakeholders workshop.
The workshop was attended by representatives of TRAGSA, BFK, Aitex, Textil Santanderina and Motorola. Comments and feedback provided by stakeholders were obtained during the workshop and filled after the event in a form of a survey. Information collected has been divided depending on the topic discussed in three different sections.
1. Workshop organization and engagement with SIXTHSENSE project
All the entities found the workshop interesting or very interesting, information provided was comprehensive or very comprehensive, and all participants liked the reduced group of participant and showed their interest to keep updated about the evolution of the project and the next steps.
2. Sensing and electrotactile biofeedback technologies in alpha prototype
The monitoring of physical and chemical parameters was interesting for all the stakeholders. Electrotactile biofeedback technology was very well received and it was thoroughly dicussed. The positioning of the electrotactile biofeedback technology was preferred by stakeholders to be used as an armband or to be located in the vest. The potential of the central dashboard in cases when the team coordination is required was discussed, as it could be converted in the most added value component of the SIXTHSENSE technology.
3. Sectors and fields of applications
Beside the main use intended for first responders, the core technology of the SIXTHSENSE project is envisioned so that it can be later modified or adapted in some other sectors, and as reported by stakeholders, those can be: sports sector, workers 4.0 (petrochemical and heavy industries), healthcare (monitoring of long term and low movement patients) or space work programmes and aeronautical applications.
Due date: 31.01.2023.
Actual submission date: 30.01.2023.
Partner responsible: MDS
Reviewed by: TEC
The main aim of this deliverable is to continue establishing initial engagement with SIXTHSENSE project Stakeholders.
The Beta prototype developed by the internal projects partners is shown to stakeholders identified by external project partners with the aim to receive feedback and suggestion to improve the technology of the project. The involvement of external partners will enhance the number of sectors where this innovative technology could be applied.
In this document, the main information related to the preparation and execution of Beta stakeholder workshop is reported. The event took place in a parallel session of the Nicosia Risk forum 2022 Conference with several talks and demonstration videos. The attendees of the conference were invited to participate in the workshop where smooth and fluid communication pathways were opened for the future. Main outcomes and feedback provided by stakeholders are collected and will be considered for a better implementation of the SIXTHSENSE project and stakeholder workshops for the improvement of the next Gamma prototype.
Due date: 31.10.2023.
Actual submission date: 31.10.2023.
Partner responsible: MDS
Reviewed by: TECSR
The main purpose of this deliverable is to continue to establish the engagement with SIXTHSENSE project Stakeholders. Gamma prototype developed by the internal projects partners is shown to stakeholders identified by external project partners with the object to receive feedback and suggestion to improve the technology of the project. The involvement of external partners will increase the number of areas where this new technology can be applied.
Gamma stakeholder workshop took place as part of the 4th meeting of Firefighter’ safety organized by Primorsko-Goranska County Fire Department and the Faculty of Civil Engineering of Rijeka that took place in Rijeka (Croatia) on October 20th, 2023. During the conference several talks, and demonstration videos were presented. The attendees of the conference were invited to participate in an open discussion after the presentation. Main outcomes and feedback provided by stakeholders were collected and will be considered for a better implementation of the SIXTHSENSE project.

Milos Kostic (TEC) presenting the gamma prototype in the 4th Firefighters Conference held in Rijeka October 20th,2023.

Meeting attendees listening to the presentation and filling the survey

Daniel Izquierdo (MDS, left) and Milos Kostic (TEC, right) in the booth showing the garment of the gamma prototype developed in SIXTHSENSE project
SIXTHSENSE stakeholders database, counting 85 stakeholders from alpha and beta stages, has been updated with 38 new end-user organizations. Most of the entities and participants in the event found the SIXTHSENSE presentation very interesting (60%) or interesting (35%), with more than 90 % of all attendants evaluating the technical information as understandable or very understandable. All of them thought that both sensing and biofeedback technologies developed in the SIXTHSENSE can be successfully applied in their sector. The participants suggested additional sensors (electrophysiological, electrochemical and environmental) of interest and discussed the integration into personal protection equipment. Majority of the participants considered the technology useful for other sectors, such as healthcare, sports, defense and industry 4.0.

